Link to Pubmed [PMID] – 17256986
Biomacromolecules 2007 Feb;8(2):566-72
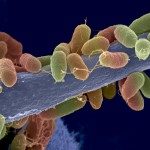
Pepsin (EC 3.4.4.1) from porcine stomach mucosa caused depolymerization of a chitosan sample (a copolymer of glucosamine and N-acetylglucosamine linked by beta-1-4-glycosidic bonds). N-terminal sequence and zymogram analyses confirmed dual (proteolytic and chitosanolytic) activities of pepsin. Optimum depolymerization occurred at pH 5.0 and 45 degrees C with an activity of 4.98 U. Low molecular weight chitosan (LMWC), the major depolymerization product, was obtained in a yield of 75-82%, the degree of polymerization of which depended on reaction time. The LMWC showed a nearly 10-14-fold decrease in the molecular mass as compared to native chitosan, which was also confirmed by GPC and HPLC analyses. IR and 13C NMR spectra indicated a decrease in the degree of acetylation (DA, approximately 13.4-18.8%) as compared to native chitosan (approximately 25.7%), which was in accordance with the CD analysis. Native chitosan had a crystallinity index (CrI) of approximately 70%, whereas there was a decrease in the CrI of LMWC (approximately 61%). The latter showed a better bactericidal activity toward both Bacillus cereus and Escherichia coli, which was more toward the former. The bactericidal activity was essentially due to the lytic and not static effect of LMWC, as evidenced by the pore formation on the bacterial cell surface when observed under SEM. This study suggests the possible use of pepsin in place of chitosanase, which is expensive and unavailable in bulk quantities for the production of LMWC of desired molecular mass that has diversified applications in various fields.