Link to Pubmed [PMID] – 16188454
Biologicals 2006 Mar;34(1):21-7
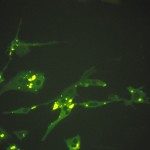
Rabies is an endemic, fatal zoonotic disease in the developing countries. Prevention and post-exposure therapy require safe and efficacious vaccines. The vaccine potency depends on the amount of immunogenic rabies viral glycoprotein antigen in the vaccine preparation. In order to estimate the rabies viral glycoprotein antigen, a specific monoclonal antibody was developed and used in an immuno-capture ELISA (IC-ELISA). The monoclonal antibody binds a conformational epitope on the natively folded rabies viral glycoprotein as indicated by specific, membrane fluorescence on unfixed, rabies virus infected murine neuroblastoma (MNA) cells and glycoprotein gene encoding plasmid transfected COS cells. In addition, the monoclonal antibody competes with and blocks a glycoprotein antigen site III binding monoclonal antibody (mAb-D1, Institut Pasteur, Paris, France). The monoclonal antibody was used in an IC-ELISA using an in-house standard to quantify the rabies viral glycoprotein antigen in 12 vaccine preparations with potency values ranging from 4 to 18 IU. The results indicated a good correlation with the NIH mouse potency assay (r=0.83). The immuno-capture ELISA described in this study can be used to quantify the immunogenic rabies viral glycoprotein antigen in the inactivated rabies viral antigen preparation in a simple and rapid format, which enables better vaccine formulation.