About
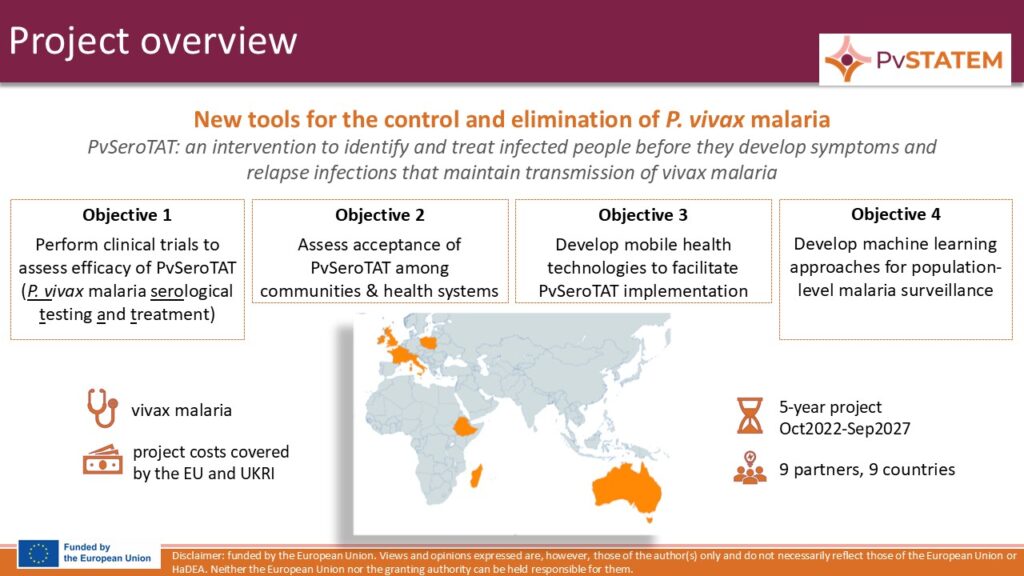
Malaria elimination requires elimination of all malaria species. Although there has been tremendous progress in the development of new interventions for P. falciparum malaria, the goal of malaria elimination cannot be considered achievable unless there is also comparable innovation for interventions specifically targeting P. vivax malaria. The P. vivax Serological Testing and Treatment in Ethiopia and Madagascar (PvSTATEM) project aims to address this challenge by developing a new intervention for the control and elimination of P. vivax malaria.
P. vivax malaria is resilient to elimination efforts due to its ability to form dormant liver stages (hypnozoites). These liver stages cannot be detected and can reactivate weeks to months after the initial infection causing relapse infections. Blood-stage parasites arising from relapses can cause illness and allow onwards transmission from humans to mosquitoes.
We have developed a diagnostic test to measure antibodies to a selection of P. vivax antigens that identifies individuals who have been recently infected, and thus likely to carry hypnozoites. These individuals can be targeted for treatment with primaquine to clear hyponzoites and prevent relapse infection. This combination of a novel diagnostic test and primaquine treatment provides a new intervention for malaria control and elimination: P. vivax Serological Testing and Treatment (PvSeroTAT).
The overall objective of PvSTATEM is to implement a cluster-randomized trial in Ethiopia and Madagascar to demonstrate the effectiveness of a new anti-malaria intervention based on P. vivax serological testing and treatment (PvSeroTAT) with primaquine to prevent the relapse infections responsible for maintaining P. vivax transmission. In parallel, the consortium will evaluate community acceptability of PvSeroTAT, innovate mobile technologies for efficient implementation of PvSeroTAT (mHealth approach), and develop machine learning approaches for population-level malaria surveillance.
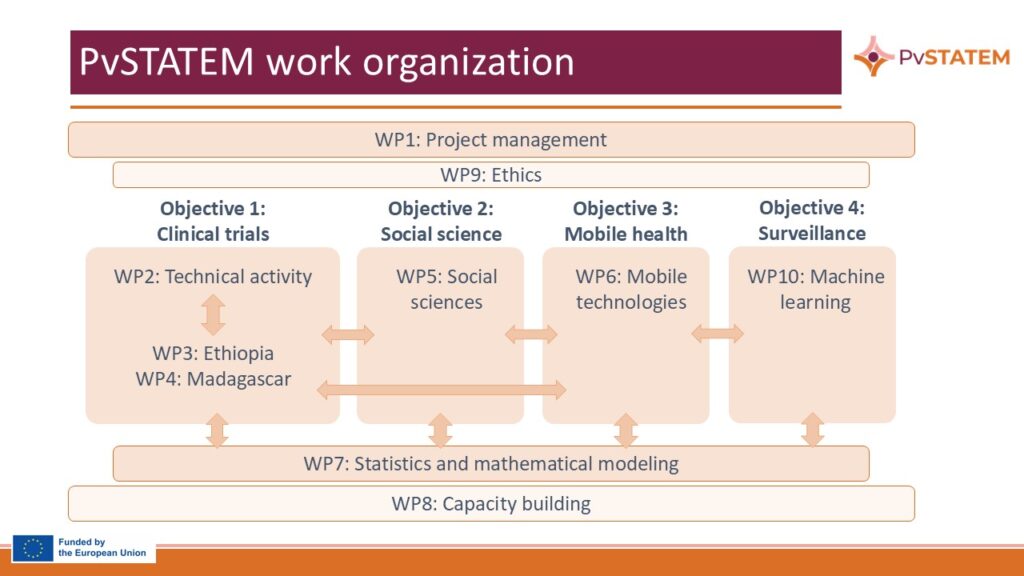
Project structure
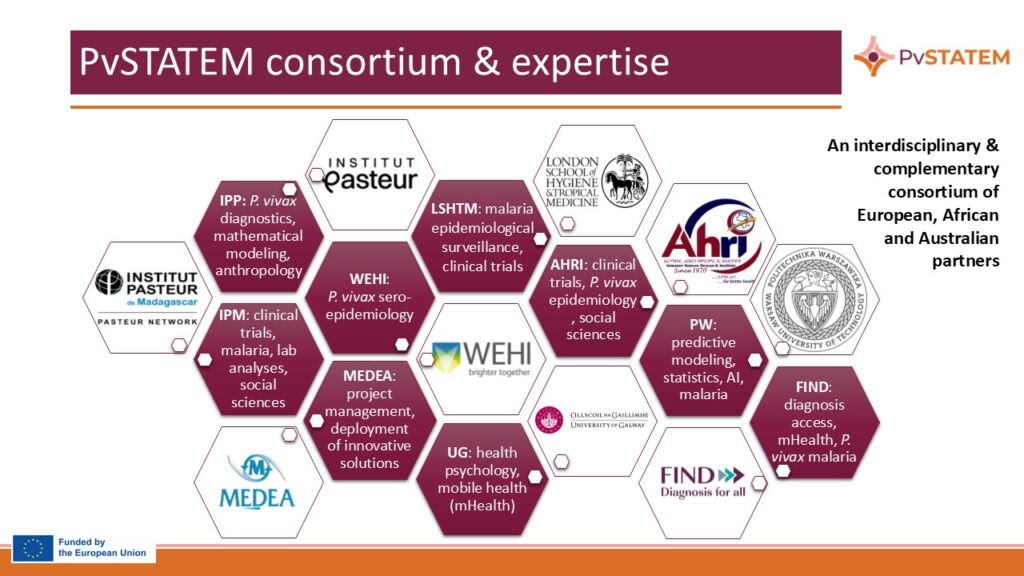
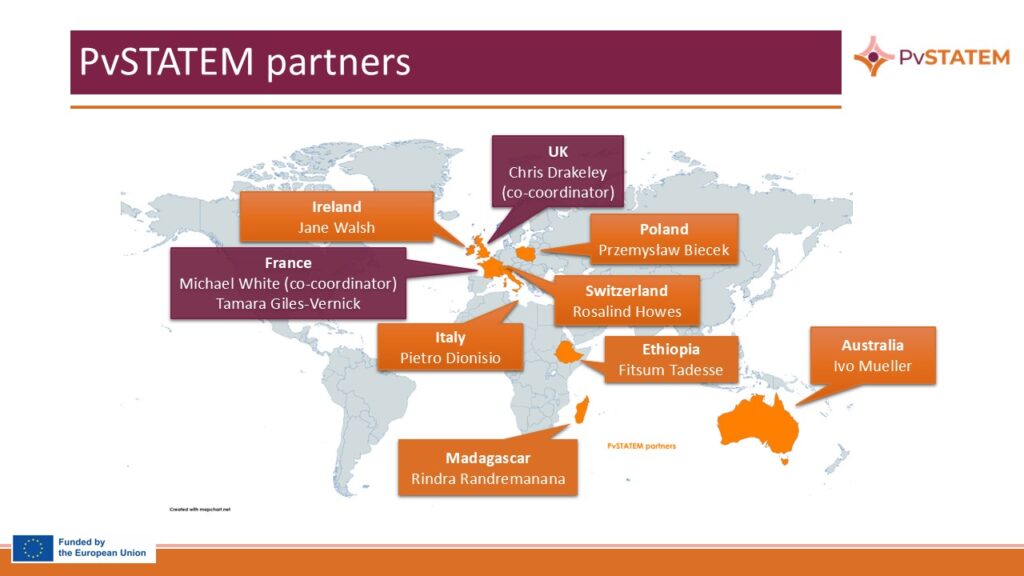
Consortium
News
To read more about the project, the consortium, and the latest updates visit our website.
Funding