Link to Pubmed [PMID] – 17118469
Mol. Biochem. Parasitol. 2007 Jan;151(1):89-99
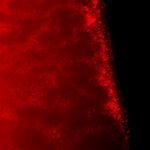
We have previously identified a number of DBLgamma domains in Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) transcripts obtained from placental parasite isolates, showing that they bind specifically to chondroitin sulfate A (CSA) (Khattab A, Kun J, Deloron P, Kremsner PG, Klinkert MQ. Variants of Plasmodium falciparum erythrocyte membrane protein 1 expressed by different placental parasites are closely related and adhere to chondroitin sulfate A. J Infect Dis 2001;183:1165-9). Here we give a more detailed physico-chemical and binding characterisation of the soluble, recombinant DBLgamma domain derived from one of these isolates. Results from circular dichroism and limited proteolysis experiments are consistent with the recombinant domain being expressed with the native fold. Specific binding of DBLgamma to placental cryosections was demonstrated by labeling with antibodies raised against the recombinant domain; binding was diminished after treatment of the cryosections with chondroitinase or by blocking with anti-CSA antibody, showing that CSA mediates the interaction. Binding of the DBLgamma domain to purified placental chondroitin sulfate proteoglycan (CSPG) was also studied using surface plasmon resonance techniques, with DBLgamma as analyte and CSPG immobilised on the sensor chip; these quantitative measurements gave an affinity constant in the mu-molar range under the conditions used. The native conformation of the DBLgamma domain is essential for CSPG recognition since binding to the sensor chip is abolished when the protein is irreversibly reduced. As with the placental cryosections, association was significantly reduced after treating the immobilised CSPG with chondroitinase. Together, these results demonstrate specific interaction between the DBLgamma domain and the placental receptor.